The First Flu Shot
By James Tobin
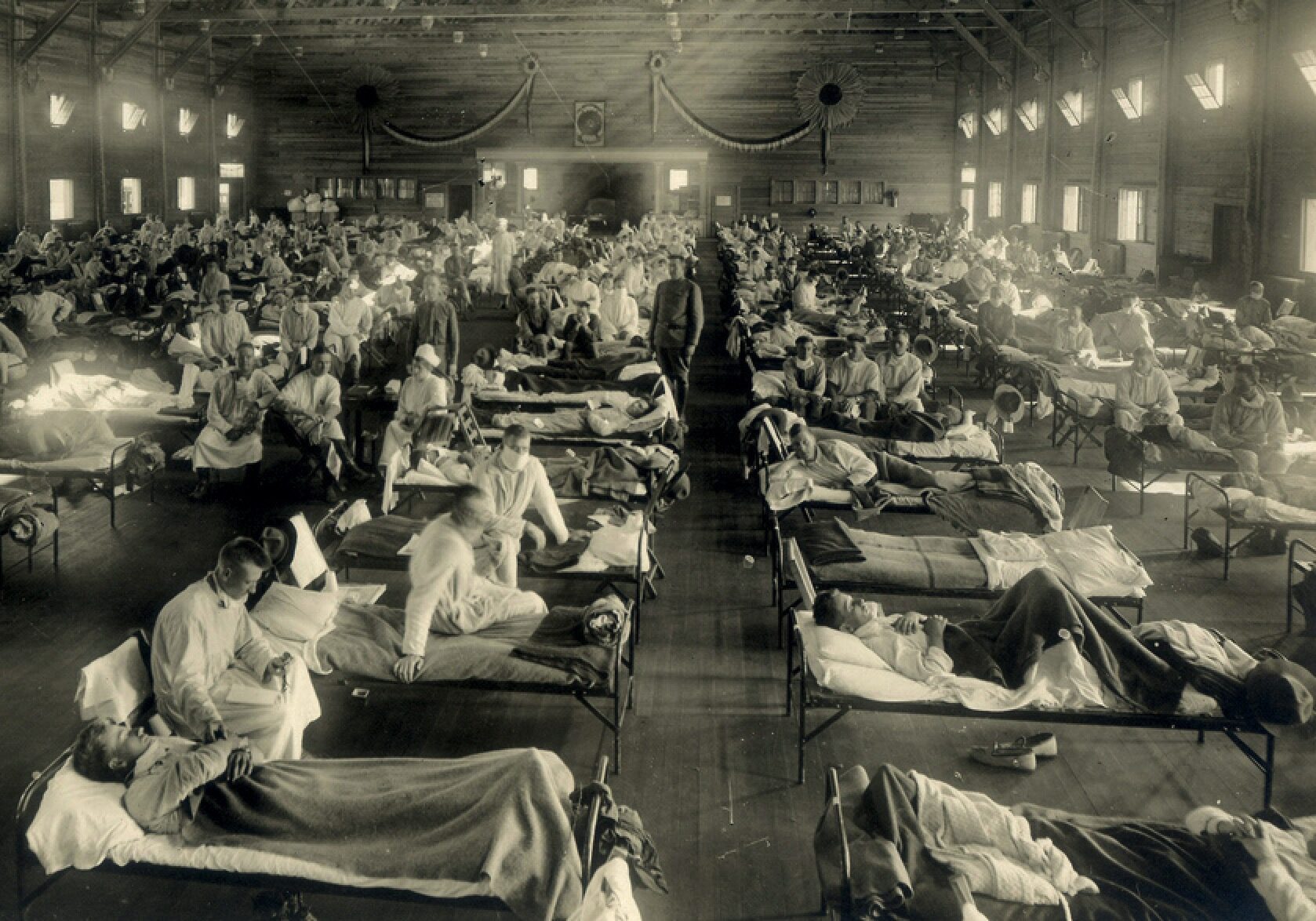 enlarge
enlarge
Caption
Incontrovertible evidence is now available that the resistance of man to infection by the viruses of influenza A and B can be enhanced.– Tommy Francis and Jonas Salk
-
Chapter 1 The Ghosts of 1918
Early in 1941, a medical scientist named Thomas Francis moved his young family from New York to Ann Arbor in a hurry, the way he did everything.
Recently he had turned down job offers from Harvard and Columbia. But when Michigan came calling, he said yes. His mission would be to start a brand new department devoted to the prevention of epidemic diseases. It was a rare chance to make a major mark in his field.
He got straight to work on a dozen tasks at once — hiring faculty; organizing labs; seeking funding; planning courses; pushing his own research along.
Then another call came, this one from the Department of War in Washington, D.C.
Americans were facing the growing prospect of being pulled into war on both sides of the world. Isolationists were against it, but the possibility of staying at peace was dwindling by the week. The U.S. House of Representatives had approved President Franklin Roosevelt’s call for a military draft by the margin of one vote, and now some 16 million young men were registering for service.
In Washington, graying men with advanced degrees in medicine and microbiology were meeting in hushed rooms away from the press. Their business was quieter but no less urgent than turning out weapons and training soldiers.
They were mulling over memories of overcrowded hospital wards in the winter of 1918-19, when the deadliest epidemic in history had circled the globe. In those months lethal strains of influenza killed at least 50 million people, perhaps 100 million, more than the medieval Black Death killed in a century.
No one then had known exactly what caused it, still less how to prevent a recurrence. The Italian word influenza derived from a Latin word for the influence of the stars on human affairs — a grim visitation beyond anyone’s control.
Before 1918, it had come and gone in cycles, a serious illness, especially for the elderly, but nowhere near as deadly as smallpox, yellow fever or cholera. The 1918 variant was far worse. The usual symptoms of headache, fever and muscle aches swiftly turned to severe pneumonia. Patients coughed up blood and suffocated. Autopsies revealed lungs turned blue from lack of oxygen. Especially vulnerable were adults in their late teens and early 20s — the age of soldiers.
In the 1918-19 pandemic, influenza and its related illnesses killed nearly 50,000 American servicemen, almost as many as had died on the European battlefields. Crude vaccines were tried, but none worked. A similar infection in this new war would find millions of young Americans crammed into barracks and troopships. The impact would be unthinkable.
So Henry L. Stimson, the U.S. secretary of war, appointed major figures in medical science to a Board for the Investigation and Control of Influenza and Other Epidemic Diseases in the Army. They, in turn, authorized a specialized Commission on Influenza.
The board’s task was of the highest urgency. They must find a physician capable of preventing a recurrence of 1918-19.
The president of the board, Dr. Francis G. Blake, dean of the Yale School of Medicine, gave his colleagues the name of a student he had come to know well 20 years earlier: Tommy Francis.
Patients coughed up blood and suffocated. Autopsies revealed lungs turned blue from lack of oxygen.
-
 enlarge
enlarge
Caption
Francis gained his original expertise as a lab researcher, but as an epidemiologist, he turned to the compiling of data.Chapter 2 Microbe Hunter
Born in 1900 in Gas City, Indiana, Francis was the son of Welsh immigrants. His father was a pious steelworker, his mother a Salvation Army nurse. The Christian duty to help the poor and the sick ran strong in the family.
In New Castle, Pennsylvania, where the Francises moved for a steel-mill job, Tommy went around town with the local doctor, who showed him the structure of blood cells through the lens of a microscope. He was fascinated.
Outgoing, tough and stocky, Francis was a boxer at Allegheny College. In 1921 he started at Yale Medical School, which had recently revamped its curriculum. It was now one of the nation’s best, paying close attention to scientific research and fully embracing clinical education — that is, placing students in direct daily contact with professors as they treated patients.
At Yale, Francis was so good at basic research that a professor urged him to get further training at the prestigious Rockefeller Institute in New York. Arriving on a Sunday, Francis found the gates locked. He threw his bag over the fence and climbed in after it.
For 10 years at Rockefeller he hunted microbes in the lab. He became an expert on pneumococcus, the bug that triggers bacterial pneumonia. At the same time he was so adept at treating patients that he became the favorite doctor of the Rockefeller family.
The competing instincts of the healer and the scientist warred inside him. One of his mentors, Dr. Oswald Avery, a brilliant molecular biologist, once found Francis with his head down on his research bench.
“What’s the matter, boy?” Avery asked. Francis said he was sorry another pneumonia season was about to start. He would have to abandon his test tubes to care for patients. “What’s in these flasks is much more exciting,” he said.
Avery brought him up short. Francis must be a physician first, a scientist second, the older man said. He must not forget that the only reason for the flasks was “to lick the pants off the pneumococcus.”
Then, in 1933, British microbiologists isolated the virus that causes influenza in humans. Francis quickly confirmed their findings. This was big. Memories of the 1918-19 pandemic were still raw. Viruses, far smaller than bacteria, were barely understood, and virology was a new science. But if Francis could help to uncover the secrets of this scourge, the cause of so much sorrow, he might light a path toward the ultimate goal — a vaccine.
-
 enlarge
enlarge
Caption
The English physician Edward Jenner is credited with one of the first modern vaccines, which he developed after observing that milkmaids who had endured cowpox developed immunity to smallpox, a related disease. Jenner is depicted here in the act of vaccinating his own child, thanks to the milkmaid who stands by.Image: Wikimedia CommonsChapter 3 The Vaccine Idea
Medical scientists grasped that the strongest weapon against infection was the human immune system, the army of cells that are provoked into action by invading germs. Against the most lethal diseases, immune cells often lost the battle and the human host died. But if the host survived, the immune cells triggered by that battle remained in the bloodstream, ready to ward off any new attack by the same germ.
The vaccine idea was this: If you gave a person a weakened form of a particular germ, you could spark the immune system into action without threatening the patient. Then those immune cells would stay active in the person’s body, ready to fight off any new infection by that particular germ if it got into the body at its full strength.
So far, vaccines had been tried against only a few infectious diseases — smallpox, diphtheria, tetanus and pertussis (whooping cough) — and not in large numbers. But Francis and a few other medical scientists were pondering the possibilities of vaccination on a massive scale against a broader array of pathogens.
Because of 1918-19, influenza led the list.
To have any chance at a vaccine, medical scientists had to know everything about the invading microbe — its structure; its life cycle; where it lived outside the human body; how it was transmitted from one body to another; how it flourished once it got inside; and how it fought with immune cells.
Viruses were many times smaller than bacteria, so they were harder to study. Scientists didn’t even know if viruses were living organisms or more like tiny machines. (The answer, even now, is: Both, sort of.)
Still just in his 30s, Francis became the country’s leading authority on influenza. He was the first to show that flu viruses changed in their chemical structure, deceiving the human immune system. In other words, the virus was not a stationary target. It was a shapeshifter. A person who had already had the flu would carry an immunity to the strain that had made them sick. But a new strain could be just different enough in its chemical makeup that the person’s immune cells — the ones stimulated by the original infection — wouldn’t recognize this new intruder.
The implication for medical sleuths like Francis was daunting. They would not be able to curb influenza with a single vaccine because it was not really a single virus. Any effective vaccine would have to carry weapons against more than one strain. Not that a vaccine against even one strain would be easy. Francis had developed a primitive vaccine that showed promise in the lab, but it was nowhere near ready for general testing. Attempts at flu vaccines elsewhere were inconclusive.
That was where things stood in 1939 and 1940, as Americans faced the prospect of being drawn into war.
Francis had recently left the Rockefeller Institute to become chair of microbiology at New York University.
Then, early in 1941, he was contacted by Henry Frieze Vaughan, an epidemiologist who had just left his post as Detroit’s public health commissioner to become dean of U-M’s new School of Public Health. He soon had Francis’s agreement to start a Department of Epidemiology.
With funding from the National Foundation for Infantile Paralysis, Francis was getting ready to study the possibilities of a vaccine for polio. That was when the Army tapped him to direct its Commission on Influenza.
When the Japanese attacked the Pacific fleet at Pearl Harbor in December 1941, his assignment took on the highest urgency. Besides running his department at U-M, he must now advise the Army on healthy housing and sanitation at military camps across the country and overseas; watch out for and treat flu outbreaks; and see to his main task — to devise, develop, test, manufacture and administer a vaccine capable of preventing a recurrence of the 1918-19 pandemic.
He would need a lab staffed by the best young microbiologists he could find.
Tommy Francis was the first to show that flu viruses were shapeshifters deceiving the human immune system.
-
Chapter 4 A “Pleasantly Energetic” Young Man
In his time at NYU, Tommy Francis had taken notice of a medical intern named Jonas Salk. He was bright, hard-working and deeply interested in research. He showed his aptitude in the lab by cutting the lungs out of mice infected with influenza, then painstakingly extracting specimens of the influenza virus from tiny pieces of lung tissue.
“He turned out to be extremely able,” Francis recalled later. “There was something appealing about the pleasantly energetic way in which he kept impressing you with his desire to do research.”
When Francis left New York for Ann Arbor, Salk had written immediately to ask him for a research post. But in the rush of starting his new job, Francis failed to write back.
Then, four days after Pearl Harbor, Salk wrote again, saying: “I feel my greatest contribution now is in utilizing the training I had with you, since it is in a field that has such special implications and significance in war time.”
Francis urged him to finish his internship first, but Salk came west anyway, whereupon Francis got him a grant of $2,100 and a draft deferment, telling the Army that Salk’s service in the lab was critical to the war effort. The young doctor, just 27, was soon in charge of the day-to-day work on influenza.
The work would demand enormous patience, skill and scientific ingenuity. In the era before electron microscopes, the flu virus could not be seen, stained or cultivated in test tubes. It was hard even to be sure a given patient actually had influenza, since other microbes caused similar symptoms. Researchers had been raising crops of the virus by injecting the throat washings of human flu patients into ferrets, then more ferrets, then finally into mice, a tricky and time-consuming process. Now a better way had been found — by cultivating the virus in the embryonic fluid of chicken eggs — but this, too, was painstaking work.
“Jonas was a blessing in those days,” Francis said later. “Workers with his initiative and talent were even scarcer than usual because of the war… It was a busy time, the pressure was on, and Jonas fit right in.”
Francis was a fierce taskmaster. Forever asking questions, scribbling notes in a small notebook, scrutinizing underlings over half-moon glasses, “Tommy could be tough, hard, and could drive people sometimes beyond what they thought they could bear,” a colleague recalled. “But everyone knew that he never drove anyone else harder or farther than he would drive himself. Everyone knew that Tommy would be as pitilessly critical of the work of his most senior colleague as he was of the most junior associate.”
Week by week, results began to emerge.
It was a busy time, the pressure was on, and Jonas fit right in.
– Tommy Francis -
 enlarge
enlarge
Caption
A globular influenza virus bumps into a respiratory cell in the nasal passages. Tiny branches reaching upward from the cell bind with proteins on the virus's surface like a key in a lock. Next the virus will burrow inside the cell and make copies of itself. The copies will spread through the host's system, and a flu infection will be underway.Chapter 5 Broken Eggs
Many virologists had been pessimistic about making a flu vaccine with a virus that had been weakened (“attenuated”) or killed, whether by chemicals or radiation. Deprived of its virulence, it simply wouldn’t work, they said. Instead, they theorized about using a living flu virus. Perhaps it could be modified just enough to trigger the immune system without making the patient fatally ill. But the Army wanted no part of that, fearing that a live virus used in a vaccine might mutate and cause a new pandemic.
Francis believed a “killed-virus” vaccine could work. So did Salk. Francis designed their strategy, launched Salk and the other investigators at the task, then oversaw their progress as he traveled the country to oversee the Army’s health precautions and consulted with the War Department.
Step by step, they began to probe more deeply into the microscopic interactions of the influenza virus and the immune system. “What had until then been a purely empirical approach — essentially in the tradition of the art of making things — began to develop into a science,” Salk later wrote. “Not only was it of interest to know whether or not something ‘worked’ but it was of equal interest to know why or how it worked.”
They learned that a flu virus rendered harmless by diluted formaldehyde could indeed prompt an immune reaction as powerful as the response triggered by a full-strength virus. But they also learned just how hard it would be to make a vaccine capable of fighting the virus’s confounding ability to appear in shifting types and subtypes.
One of Salk’s biographers, Charlotte Decroes Jacobs, described the patience and dexterity required to create a sample vaccine to test.
Salk would pick up an 11-day-old fertilized chicken egg, spray the shell with germicide, prick the shell with a needle, then inject the sac around the embryo with a drop of solution in which the virus was suspended. Two days later he would burn the top off the shell with a miniature blow-torch, then siphon fluid from the sac. He had detected that viral cells clung to red blood cells; this made it easier to locate the virus in the fluid. He would pull out a tiny pellet of blood-cells-plus-virus, then wash off the blood cells, yielding a pure viral specimen. This Salk would hit with a dose of chemicals to sap its virulence without destroying it. Then he dropped it into a sterile vial.
“This was the influenza vaccine,” Jacobs wrote, but only if all went well. “Salk faced innumerable pitfalls: scores of broken eggs, a pellet that disintegrated upon removal, [and] a vaccine that still contained live virus” — a virus that might just give him a dangerous illness.
Less than a year after they started, Francis could tell the War Department he had a vaccine ready to test.
-
 enlarge
enlarge
Caption
The Eloise Hospital in western Wayne County in the 1940s, when it was the site of trials of U-M's influenza vaccine.Chapter 6 Trials Without Consent
In 1942 there were no standard protocols, much less laws, requiring medical scientists to obtain the informed consent of people participating in trials of new medicines. Such rules were put in place only after World War II, when it became known that German doctors had performed medical experiments on defenseless Jews and other prisoners in Nazi concentration camps. In the United States of the 1940s, it was still standard practice to test new drugs on institutionalized patients without their consent.
So on the eve of flu season in the fall of 1942, the Commission on Influenza took its vaccine to test on 8,000 psychiatric patients at two hospitals — the Eloise Mental Hospital operated by Wayne County west of Detroit, and Ypsilanti State Hospital. Salk oversaw the process.
“One only can guess,” Charlotte Jacobs wrote, “that Salk felt the prospect of fifty thousand or more young soldiers dying from influenza justified their actions.”
Investigators drew blood from each participant, then inoculated them with the trial vaccine. Two weeks later, they came back to draw another blood sample from each.
They found the level of flu-fighting immune cells had risen in 85 percent of the subjects — an impressive result.
But there was no flu outbreak that fall or winter. So the researchers returned to Ypsi State in the spring of 1943. They would have to give subjects the flu themselves.
They selected 200 male patients. Some had been vaccinated the previous fall; some hadn’t. To half they gave a saline placebo. To the other half they gave the full-strength influenza virus, spraying a mist made from the dried lung tissue of infected mice into the subjects’ nostrils.
It was a double-blind trial, meaning that neither the researchers nor the subjects knew which men had been given the virus and which the placebo.
For the next two weeks, hospital staff checked the subjects for symptoms. Some came down with the flu, some didn’t.
Then Francis and Salk broke the double-blind code.
It wasn’t a perfect result, but it was significant. Of those who had received the vaccine, only 16 percent developed the flu. Of those who hadn’t, nearly half got sick.
So the vaccine, though hardly infallible, was proving effective in strictly controlled tests.
Would it help to prevent an actual outbreak in the real world?
-
 enlarge
enlarge
Caption
Twelve thousand trainees like these in U-M's branch of the Army Specialized Training Program were treated with the influenza vaccine developed by Francis and Salk.Chapter 7 “Incontrovertible Evidence”
Looking toward the 1943-44 flu season, Francis and Salk geared up for major trials in the field. They arranged to test 12,500 subjects, all of them participants in the Army Specialized Training Program at eight universities, including Michigan, and five medical schools. They enlisted three drug firms to manufacture doses of the vaccine, this time with two of the strains categorized as Type A, one of Type B. Again the test was double-blind. Half were vaccinated, half got the placebo.
Francis was overseeing the influenza testing while scrambling to keep tabs on the Army’s health all over. He went to North Africa and Italy to consult on a severe outbreak of hepatitis among U.S. troops; to London on the eve of the D-Day invasion; back to the U.S. to visit more Army camps that summer.
Autumn came. Francis’s network began reporting influenza cases at the test sites — 17 at U-M, then 100 at St. Louis University. Soon it was clear that a major flu epidemic was underway, the most widespread since 1918, though this year’s strain didn’t attack the lungs so there were few fatalities.
The flu investigators began to tabulate the results. When the counting was done, they discovered that only two percent of subjects who had been vaccinated had gotten sick. And they saw evidence of a “herd effect.” That meant that in U-M dorms, for example, the incidence of the flu was considerably lower than it was in communities where no one had received the vaccine. There were simply fewer people with the flu, so fewer people caught it.
* * *
In 1944, acting on the advice of the Commission on Influenza, the surgeon general gave orders to inoculate every member of the U.S. Army in the fall of 1945. By then the war was over, but millions of Americans were still in uniform and sharing the close-packed quarters where infections easily spread. All were vaccinated.
An epidemic of Type B influenza struck that fall. Of the vaccinated soldiers, only eight percent got sick.
In the laconic style of the academic scientist, Francis and Salk released an understatement that was hardly a declaration of victory, but still carried a hint of pride: “Incontrovertible evidence is now available that the resistance of man to infection by the viruses of influenza A and B can be enhanced.”
-
 enlarge
enlarge
Caption
On April 12, 1955, Francis and Salk appeared together at a press conference in Rackham Auditorium to announce the success of Francis's field trials of Salk's polio vaccine.Chapter 8 Two Giants
In Tommy Francis and Jonas Salk, the Army had two medical scientists as good, perhaps, as any in the world — both brilliant, both utterly devoted to the work at hand, and both deeply ambitious on behalf of their own way of seeing scientific problems.
But one worked for the other. That could last for the duration of the war, maybe longer. But anyone who knew both men could tell it wasn’t going to last forever.
Salk was often a maverick in the lab, trying innovations that Francis thought unwise. Francis was a classic academic scientist, Salk something of a natural philosopher. “Damn it all, Salk,” Francis would say, “why can’t you do things the way everyone else does?”
Once Salk asked Francis to review the manuscript of a scientific article he had just drafted for publication.
Francis, an arch-stickler for precision in scientific matters, read the draft, then told Salk his conclusions weren’t supported by his evidence.
Salk said: “The inferences were warranted by reason, if not by hard data.”
Francis said that wasn’t the way U-M’s Department of Epidemiology did things. Salk said he supposed he’d send the article out for publication anyway.
“If you do,” Francis said, “you’d better go with it.”
Salk stayed. But he was increasingly frustrated by Francis’s high profile while he, Salk, labored on in the lab, unrecognized by the scientific establishment.
“Jonas was doing all the work and Tommy was getting all the fame,” said a colleague of both, “so that caused an obvious friction.”
When the war was over and the first big Army vaccination was complete, Salk applied for openings at the University of California and Western Reserve University. No offer came. Then, in 1947, he went for a talk with the new dean of medicine at the University of Pittsburgh. It was no great medical school, but the dean had big plans and wanted Salk to help achieve them.
Salk accepted Pitt’s offer. He would build a new program in virology.
“Tommy Francis thought I was making a mistake,” he said later. “So did everyone else. I can remember someone asking me, ‘What’s in Pittsburgh, for heaven’s sake?’ and I answered, ‘I guess I fell in love.’ What I was in love with, of course, was the prospect of independence.'”
* * *
Seven years later, Jonas Salk had developed a vaccine for polio, a disease even more dreaded than influenza, since it killed and crippled children in savage summertime epidemics that were growing worse by the year.
To be proven effective, Salk knew, his new vaccine must be tested in the largest field trials ever attempted, all in the glare of worldwide publicity. And they must be conducted with absolute integrity, or his breakthrough would fall to pieces.
The bill for the trials was to be paid by the National Foundation for Infantile Paralysis, which had underwritten Salk’s pursuit of the vaccine. Critics balked. The Foundation had too great a stake in the outcome, they said. How could it guarantee an unbiased result?
So the Foundation asked Tommy Francis to design and oversee the trials. His appointment laid to rest any questions about bias and integrity.
As a model, Francis used the influenza trials conducted with Army trainees on college campuses. He resisted pressure to complete the trials before the onset of the summer polio season of 1954. And he insisted on a double-blind trial. Nearly two million children, ages six to nine, took part.
This time, the principle of informed consent prevailed. The parents of every child signed their approval.
On April 12, 1955, a decade to the day after the death of Franklin Roosevelt, whose polio case had led to the campaign for a cure, Salk and Francis stood shoulder to shoulder on the stage of the Rackham Auditorium in Ann Arbor and announced the results of the polio field trials.
“SALK POLIO VACCINE PROVES SUCCESS; MILLIONS WILL BE IMMUNIZED SOON,” proclaimed the front page of the next day’s New York Times. The accompanying photo shows Salk and Francis, both smiling broadly, Salk slightly upstage of his old boss.
* * *
Polio was virtually eradicated in the United States and around the world by a combination of Salk’s injected “killed-virus” vaccine and its successor, an attenuated live-virus vaccine developed by Albert Sabin of the University of Cincinnati. (Taken orally instead of by injection, the Sabin vaccine proved to be easier to give and longer-lasting than the Salk vaccine.) The disease has mounted a comeback in places where parents refuse to allow their children to receive the vaccine.
Meanwhile, influenza marches on. In 2017-2018, it was blamed for the deaths of nearly 80,000 Americans, the largest toll in many years.
The U.S. Centers for Disease Control and Prevention recommend that virtually every American over the age of six months receive an annual vaccine to guard against the flu strains expected to circulate in the U.S. in the coming year. The vaccine is estimated to prevent infection in about two-thirds of those who receive it.
But in 2018, nearly half of Americans surveyed said they planned to skip the shot.
Sources included “Thomas Francis, Jr.: An Appreciation” by Myron E. Wegman; “The Restless Spirit of Thomas Francis, Jr., Still Lives” by Jonas Salk; “Thomas Francis, Jr., as a Clinician, 1900-1969” by John R. Paul; and “Thomas Francis, Jr., M.D. 1900-1969” by Colin M. Macleod, in Archives of Environmental Health; Jonas Salk: A Life by Charlotte Decroes Jacobs; Breakthrough: The Saga of Jonas Salk by Richard Carter; Thomas Francis, Jr., 1900-1969: A Biographical Memoir, by John R. Paul; “DeKruif’s Boast: Vaccine Trials and the Construction of a Virus,” by John M. Eyler, Bulletin of the History of Medicine; “Vaccine Innovation in World War II,” by Kendall Hoyt, Journal of Public Health.
Jonas was doing all the work and Tommy was getting all the fame, so that caused an obvious friction.
– A colleague


